Gut/Brain/Liver Axis
The Pillars of the Metabolic Matrix (Support the Brain, Protect the Liver, and Feed the Gut) are supported by a growing body of research on the axis intersecting these organs that play a fundamental role in metabolic health.
What is the gut-brain-liver axis?
The axis connecting the gut, brain, and liver, which is otherwise known as the gut-brain-liver axis, is a three-way interaction that has recently garnered increasing scientific interest. Over the last two decades, researchers have achieved significant progress in exploring gut-brain-liver communication by better understanding its development process and expanding therapeutic options. Interventions based on the gut-brain-liver connection could facilitate personalized treatment.
This page provides highlights and links to a number of studies in this area.
Research Highlights on the Brain/Gut/Liver Axis
The gut-liver-brain axis is a three-way highway of information interaction among the gastrointestinal tract, liver, and nervous systems. In the past few decades, breakthrough progress has been made in this axis, mainly through understanding its formation mechanism and increasing treatment strategies.
Source: Gut liver brain axis in diseases: the implications for therapeutic interventions, Signal Transduction and Targeted Therapy, by Mengyao Yan, Shuli Man, Benyue Sun, Long Ma, Lanping Guo, Luqi Huang & Wenyuan Gao
The gut-brain axis is a bidirectional information interaction system between the central nervous system (CNS) and the gastrointestinal tract, in which gut microbiota plays a key role. The gut microbiota forms a complex network with the enteric nervous system, the autonomic nervous system, and the neuroendocrine and neuroimmunity of the CNS, which is called the microbiota-gut-brain axis. Due to the close anatomical and functional interaction of the gut-liver axis, the microbiota-gut-liver-brain axis has attracted increased attention in recent years. The microbiota-gut-liver-brain axis mediates the occurrence and development of many diseases, and it offers a direction for the research of disease treatment.
Source: Role of gut microbiota via the gut-liver-brain axis in digestive diseases, World Journal of Gastroenterology, by Jian-Hong Ding, Zhe Jin, Xiao-Xu Yang, Jun Lou, Wei-Xi Shan, Yan-Xia Hu, Qian Du, Qiu-Shi Liao, Rui Xie, and Jing-Yu Xu
The gut-liver-brain axis describes the bidirectional interaction between the gastrointestinal system, the liver, and the central nervous system with involvement of the gut microbiota. Regulated by the intestinal, blood–brain and gut-vascular barrier, the immune system, and the microbially derived molecules that are derived from diet, the nutrition-gut-liver-brain axis has an essential role in a number of diseases. This review will canvass some of the concepts regarding the nutrition-gut-liver-brain axis and focus on common diseases with recent evidence.
Source: Nutrition and the Gut-Liver-Brain Axis, Current Hepatology Reports, by Agnes H. Y. Ho, Sunny Wong, and Rashid N Lui
The liver plays a central role in digestion, metabolism, and immune protection from pathogens (Kalra et al., 2022). Chronic liver disease caused by various etiologies, including viral, metabolic, and autoimmune diseases can impact liver function leading to chronic injury and ultimately liver cirrhosis. The impact of chronic liver injury extends beyond local organ dysfunction, and can cause perturbations systemically affecting extrahepatic organs including the brain (Montano-Loza et al., 2012; Albillos et al., 2014; Liu et al., 2022). This liver-brain interaction can be readily observed in patients who develop hepatic encephalopathy (HE) as a complication of end stage liver disease or liver cirrhosis. Although the pathophysiology of HE is incompletely understood, it is thought that alterations in central nervous system blood flow, accumulation of neurotoxic compounds, presence of inflammatory metabolites, and excess bile acids resulting from poor liver function can mediate Central Nervous System (CNS) dysfunction (Rose et al., 2020). Furthermore, symptoms generated from altered brain function, including fatigue, depression, anxiety, sleep disturbance and loss of social interest have been observed in patients with chronic liver conditions with and without cirrhosis and overall have a detrimental effect on patient quality of life (Swain and Jones, 2019). A more granular understanding of the mechanisms governing changes in brain function in relation to liver health will provide a foundation to further develop new diagnostic and therapeutic tools that can be used to improve the care of liver patients afflicted with these debilitating symptoms. The mechanism that links liver health to brain function is thought to be mediated through various avenues within the gut-liver-brain axis (Ding et al., 2020; Blaga et al., 2021; Brescia and Rescigno, 2021; D’Mello and Swain, 2021).
Source: Avenues within the gut-liver-brain axis linking chronic liver disease and symptoms, Frontiers in Neuroscience, by Henry H. Nguyen and Mark G. Swain
The gut-liver-brain axis consists of the complex interplay between the gut–brain, gut–liver, and liver–brain axes, and is a multidirectional communication network that links the enteric, hepatic, and central nervous systems. This communication network has been extended to involve endocrine, humoral, metabolic, and immune routes of communication. Through this network, the brain affects intestinal and hepatic activities, including the activity of innate immune cells and immune effector cells (Teratani et al., 2020). Meanwhile, the gut and liver influence cognition and mental health through the regulation of microbiota and host immune responses. Wang et al. find there was a potential causal relationship between gut microbiota and attention deficit hyperactivity disorder and suggest that the gut bacteria found in this study may reduce the occurrence of attention deficit hyperactivity disorder. Hildenbrand et al. investigate predisposing and precipitating risk factors for delirium (the most common acute neuropsychiatric syndrome in hospitalized patients), and find that delirium in precipitating gastrointestinal and hepato-pancreato-biliary diseases was not associated with higher age per se, but with cognitive and functional impairment. Diet is a modulator of the microbiome and is known to impact the gut-brain axis, including its influence on acute brain injuries. Krakovski et al. show that diets and probiotics beneficially modulate immune and neuronal functions, as well as the therapeutic importance of modulation by diets and probiotics.
Source: Editorial: Gut-liver-brain axis: a complex network influences human health and diseases, Frontiers in Neuroscience by Wu Hongjin, Juehua Yu, and Ming Shi
The gut vascular barrier (GVB) represents the inner layer of defense in the multilayered intestinal barrier system that finely regulates the translocation of substances from the intestinal lumen to the systemic circulation. Alterations in gut microbiota barrier homeostasis can lead to the development of intestinal and extraintestinal disease induced by a leaky gut. Increased GVB permeability can lead to leakage of harmful molecules into the blood circulation, with consequent damage to other organs along the gut–liver–brain axis. Targeting the GVB and the active molecules that drive the vascular connection of the gut–liver–brain axis might provide additional therapeutic approaches to diseases affecting organs other than the intestine.Source: The gut vascular barrier: a new player in the gut–liver–brain axis, Trends in Molecular Medicine, by Paola Brescia and Maria Rescigno.
Recent clinical and experimental evidence has evoked the concept of the gut–brain axis to explain mutual interactions between the central nervous system and gut microbiota that are closely associated with the bidirectional effects of inflammatory bowel disease and central nervous system disorders1,2,3,4. Despite recent advances in our understanding of neuroimmune interactions, it remains unclear how the gut and brain communicate to maintain gut immune homeostasis, including in the induction and maintenance of peripheral regulatory T cells (pTreg cells), and what environmental cues prompt the host to protect itself from development of inflammatory bowel diseases. Here we report a liver–brain–gut neural arc that ensures the proper differentiation and maintenance of pTreg cells in the gut. The hepatic vagal sensory afferent nerves are responsible for indirectly sensing the gut microenvironment and relaying the sensory inputs to the nucleus tractus solitarius of the brainstem, and ultimately to the vagal parasympathetic nerves and enteric neurons. Surgical and chemical perturbation of the vagal sensory afferents at the hepatic afferent level reduced the abundance of colonic pTreg cells; this was attributed to decreased aldehyde dehydrogenase (ALDH) expression and retinoic acid synthesis by intestinal antigen-presenting cells. Activation of muscarinic acetylcholine receptors directly induced ALDH gene expression in both human and mouse colonic antigen-presenting cells, whereas genetic ablation of these receptors abolished the stimulation of antigen-presenting cells in vitro. Disruption of left vagal sensory afferents from the liver to the brainstem in mouse models of colitis reduced the colonic pTreg cell pool, resulting in increased susceptibility to colitis. These results demonstrate that the novel vago-vagal liver–brain–gut reflex arc controls the number of pTreg cells and maintains gut homeostasis. Intervention in this autonomic feedback feedforward system could help in the development of therapeutic strategies to treat or prevent immunological disorders of the gut.
Source: The liver–brain–gut neural arc maintains the Treg cell niche in the gut, Nature, by Toshiaki Teratani, Yohei Mikami, Nobuhiro Nakamoto, Takahiro Suzuki, Yosuke Harada, Koji Okabayashi, Yuya Hagihara, Nobuhito Taniki, Keita Kohno, Shinsuke Shibata, Kentaro Miyamoto, Harumichi Ishigame, Po-Sung Chu, Tomohisa Sujino, Wataru Suda, Masahira Hattori, Minoru Matsui, Takaharu Okada, Hideyuki Okano, Masayuki Inoue, Toshihiko Yada, Yuko Kitagawa, Akihiko Yoshimura, Mamoru Tanida, …Takanori Kanai
The gut–liver–brain axis constitutes a multidirectional communication network that connects the enteric, hepatic, and central nervous systems. Through the complex interplay between the gut–liver, gut–brain, and liver–brain axes, this communication network extends to involve endocrine, immune (humoral), and metabolic routes of communication. Within the network, the gut and liver affect cognitive behaviors through the host’s immune responses and the regulation of microbiota, and the brain also influences intestinal and hepatic activities. Studies in animals have shown that an impaired gut–liver–brain axis is associated with diseases such as hepatic encephalopathy, Alzheimer’s disease, Parkinson’s disease, Multiple Sclerosis, depression, and autism spectrum disorder (ASD). Source: The Gut–Liver–Brain Axis, E Scholarly Community Encyclopedia.
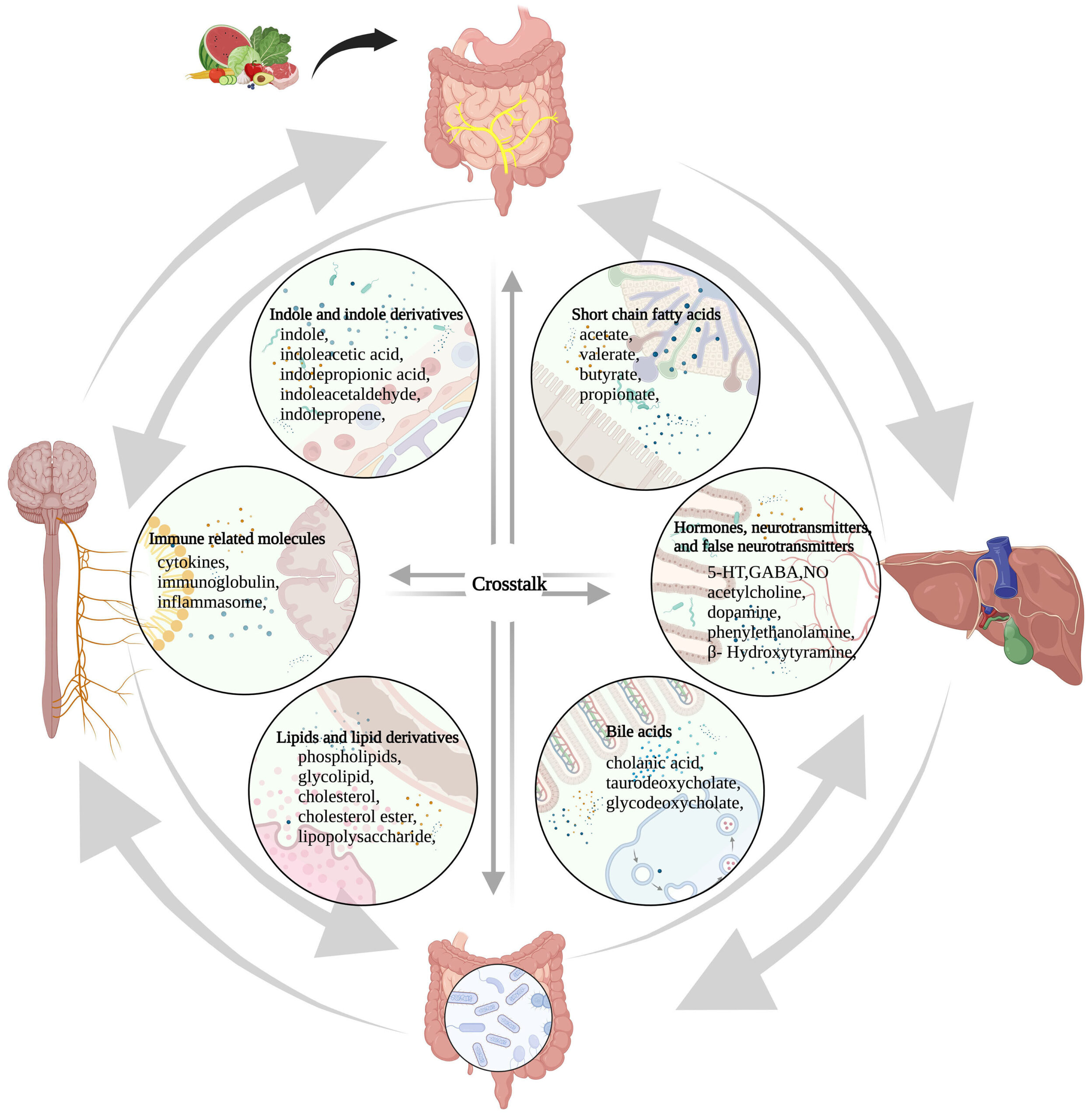
Figure 1. The microbiome-gut-liver-brain axis and molecules involved in this network. These molecules are classified into six categories, including short-chain fatty acids, hormones, neurotransmitters and false neurotransmitters, bile acids, lipids and lipid derivatives, and immune-related molecules, indole and indole derivatives. Amino acids, glucose, protein, fatty acids, vitamins, minerals, and other nutrients that are absorbed from food in the gut participate in the synthesis and conversion of these molecules directly or indirectly. 5-HT: 5-hydroxytryptamine; GABA: γ-aminobutyric acid; NO: nitric oxide.
Source: Microbiota-gut-liver-brain axis and hepatic encephalopathy
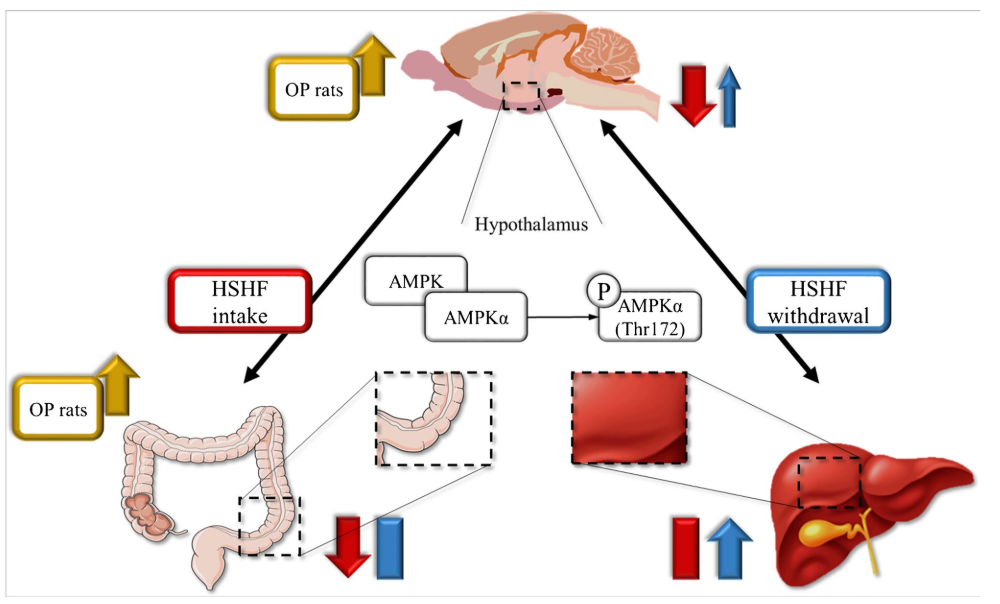
Image: Summary of fndings. AMPK phosphorylation was lowered in the colon and hypothalamus after High Sugar High Fat (HSHF) intake
Obesogenic diets (ODs) can affect AMPK activation in several sites as the colon, liver, and hypothalamus. OD intake can impair the hypothalamic AMPK regulation of energy homeostasis.
“Our results highlight the relevance in multi-organ investigations and animal phenotype evaluation when studying the energy metabolism regulations.”
Source: AMPK in the gut-liver-brain axis and its influence on OP rats in an HSHF intake and WTD rat model, Pflügers Archiv, European Journal of Physiology, DOI: 10.1007/s00424-021-02583-6
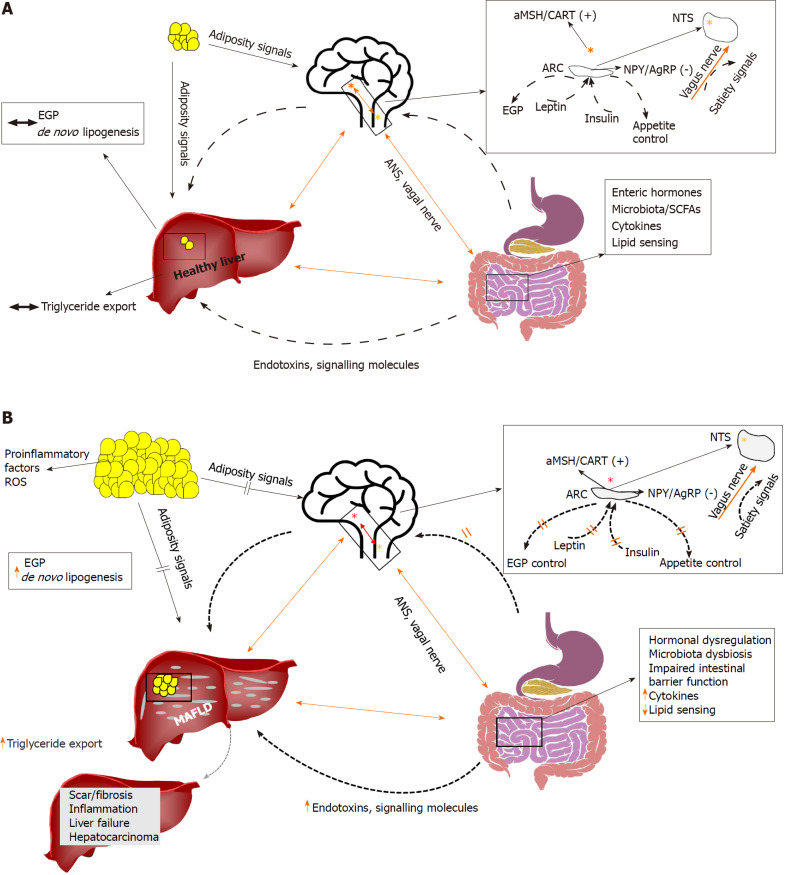
Featured Image sourced from “Brain-gut-liver interactions across the spectrum of insulin resistance in metabolic fatty liver disease,” World J Gastroenterol. 2021 Aug 14; 27(30): 4999–5018. Published online 2021 Aug 14. doi: 10.3748/wjg.v27.i30.4999
While glucose response is important, ultimately, it is insulin response and resistance that matters in terms of measuring and monitoring metabolic health. Several key reasons why are listed here:
- Insulin’s Role: Insulin is like a key that unlocks human cells, allowing glucose (sugar) from the bloodstream to enter the cell and be used for energy.
- Resistance Issues: When cells become resistant to insulin, the “key” doesn’t work as well. Glucose builds up in the bloodstream instead of being absorbed by cells, leading to high blood sugar levels.
- Long-Term Impact: Chronically high blood sugar due to insulin resistance can lead to prediabetes and eventually type 2 diabetes. It’s also linked to other health problems like heart disease and fatty liver disease.
- Brain-Gut-Liver Axis: Insulin resistance has a profound impact on the brain-gut-liver axis resulting in metabolic dysregulations linking all three organs.
So, while glucose levels give a snapshot of sugar metabolism in the blood, insulin response and resistance tell the bigger story about how efficiently the body is metabolizing sugars and fostering susceptibility to complex health issues.
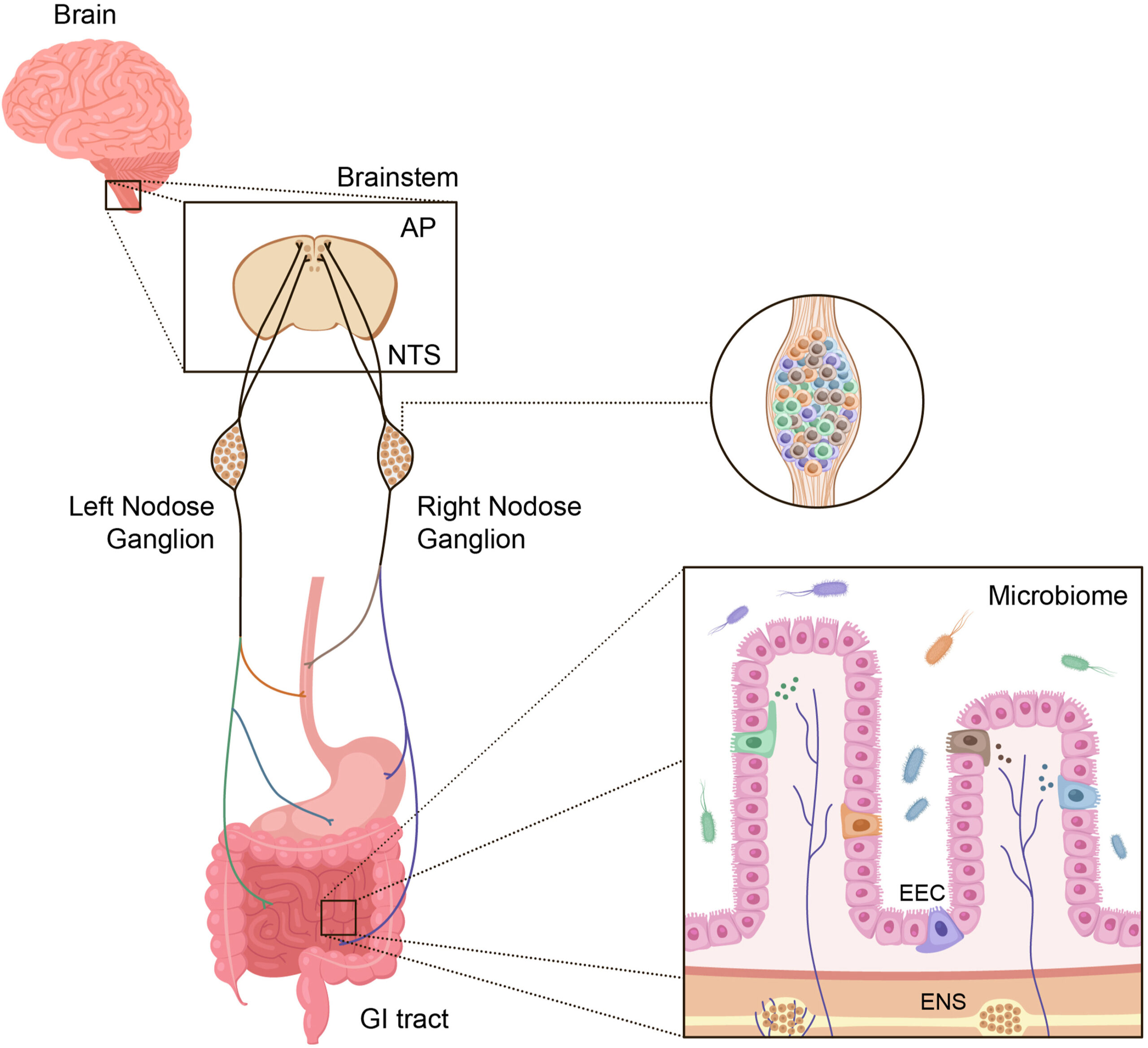
Figure 1. Major components of the gut-brain axis. The gastrointestinal tract contains the largest surface area in the body exposed to the external environment. Composed of the mouth, pharynx, esophagus, stomach, small intestine (duodenum, jejunum, and ileum), and large intestine (appendix, cecum, colon, rectum, and anal canal), its surface area is 50-200x larger than the surface area of the skin [178]. There are multiple individual components, each containing highly specialized cells, that are responsible for decoding and communicating sensory information from the gut to the brain and other organs. These include enteroendocrine cells (EECs), which produce a variety of hormones involved in both endocrine and paracrine signaling, the enteric nervous system (ENS), gut microbiota, the vagus nerve (nodose ganglia) with specific cell types that innervate discrete regions of the gut, and the central nervous system (CNS).
The gut-brain axis plays an essential role in regulating metabolism and leading therapeutics for T2DM and obesity harness this machinery.
The gut-brain axis, which mediates bidirectional communication between the gastrointestinal system and central nervous system (CNS), plays a fundamental role in multiple areas of physiology including regulating appetite, metabolism, and gastrointestinal function. The biology of the gut-brain axis is central to the efficacy of glucagon-like peptide-1 (GLP-1)-based therapies, which are now leading treatments for type 2 diabetes (T2DM) and obesity. This success and research to suggest a much broader role of gut-brain circuits in physiology and disease has led to increasing interest in targeting such circuits to discover new therapeutics. However, our current knowledge of this biology is limited, largely because the scientific tools have not been available to enable a detailed mechanistic understanding of gut-brain communication.
Source: The gut–brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders, Molecular Metabolism
An understanding of connections between gut microbiome and liver has provided important insights into the pathophysiology of liver diseases. Since gut microbial dysbiosis increases gut permeability, the metabolites biosynthesized by them can reach the liver through portal circulation and affect hepatic immunity and inflammation. The immune cells activated by these metabolites can also reach liver through lymphatic circulation. Liver influences immunity and metabolism in multiple organs in the body, including gut. It releases bile acids and other metabolites into biliary tract from where they enter the systemic circulation. In this review, the bidirectional communication between the gut and the liver and the molecular cross talk between the host and the microbiome has been discussed. This review also provides details into the intricate level of communication and the role of microbiome in Gut-Liver-Brain, Gut-Liver-Kidney, Gut-Liver-Lung, and Gut-Liver-Heart axes. These observations indicate a complex network of interactions between host organs influenced by gut microbiome.
Source: Host-microbiome interactions: Gut-Liver axis and its connection with other organs, NPJ Biofilms Microbiomes. 2022; 8: 89. Published online 2022 Nov 1. doi: 10.1038/s41522-022-00352-6

The gut-liver axis refers to the bidirectional relationship between the gut and its microbiota, and the liver, resulting from the integration of signals generated by dietary, genetic and environmental factors. This reciprocal interaction is established by the portal vein which enables transport of gut-derived products directly to the liver, and the liver feedback route of bile and antibody secretion to the intestine. The intestinal mucosal and vascular barrier is the functional and anatomical structure that serves as a playground for the interactions between the gut and the liver, limiting the systemic dissemination of microbes and toxins while allowing nutrients to access the circulation and to reach the liver. The control of microbial communities is critical to maintaining homeostasis of the gut-liver axis, and as part of this bidirectional communication the liver shapes intestinal microbial communities. Alcohol disrupts the gut-liver axis at multiple interconnected levels, including the gut microbiome, mucus barrier, epithelial barrier and at the level of antimicrobial peptide production, which increases microbial exposure and the proinflammatory environment of the liver. Growing evidence indicates the pathogenetic role of microbe-derived metabolites, such as trimethylamine, secondary bile acids, short-chain fatty acids and ethanol, in the pathogenesis of non-alcoholic fatty liver disease. Cirrhosis by itself is associated with profound alterations in gut microbiota and damage at the different levels of defence of the intestinal barrier, including the epithelial, vascular and immune barriers. The relevance of the severe disturbance of the intestinal barrier in cirrhosis has been linked to translocation of live bacteria, bacterial infections and disease progression. The identification of the elements of the gut-liver axis primarily damaged in each chronic liver disease offers possibilities for intervention. Beyond antibiotics, upcoming therapies centred on the gut include new generations of probiotics, bacterial metabolites (postbiotics), faecal microbial transplantation, and carbon nanoparticles. FXR-agonists target both the gut and the liver and are currently being tested in different liver diseases. Finally, synthetic biotic medicines, phages that target specific bacteria or therapies that create physical barriers between the gut and the liver offer new therapeutic approaches.
Source: The gut-liver axis in liver disease: Pathophysiological basis for therapy. Journal of Hepatology, 2020 Mar;72(3):558-577.. doi: 10.1016/j.jhep.2019.10.003. Epub 2019 Oct 14.
The close relationship of the liver to gut bacteria is widely recognized, studied, and constantly evolving. Evidence has shown that the alteration of the microbiome causes bacterial translocation, alteration of the composition of bile acids and increased intestinal permeability, generating the activation of several inflammatory pathways within the liver. This chronic inflammation associated with intestinal dysbiosis is believed to play a crucial role in the progression of chronic liver disease (CLD) (cirrhosis, hepatic steatosis, autoimmune diseases such as primary biliary cirrhosis, sclerosing cholangitis) and their complications (hepatic encephalopathy, hepatocarcinoma). In this review, we aim to describe the current facts of the composition of the microbiota and its effect on the development and progression of CLD, we also present the relationship of the microbiota with other pathologies (such as cardiovascular, endocrinological).
Key Content and Findings: The main facts found with strong evidence were that the colonization by the microbiota begins from birth, environmental factors are the most important for the differentiation of the microbiota, which explains the variability between individuals. Microbiota has metabolic, inflammatory and endocrinological function, and plays an important role in the pathogenesis of CLD development and progression. Hence, there still strong controversy whether dysbiosis is the etiological factor or is only a cofactor, and whether or not other microbiome component play a role in these diseases.
Source: Narrative review of gut microbiota and liver diseases: facts and fictions. Digestive Medicine Research, Vol 5 (March 30, 2022
Obesity is associated with cognitive deficit and liver alterations; however, it remains unclear whether a combination of functional foods could reverse cognitive damage and to what extent it would be associated with changes in gut microbiota and liver. With this aim, male Wistar rats were fed a high-fat-5%sucrose diet (HFS) for 4 mo. And were then fed for 1 mo. with bioactive foods. At the end of this period, liver, serum, feces, intestine, and brain samples were taken. Body composition, energy expenditure, LPS, hormones, intraperitoneal glucose tolerance test, behavioral tests, and gut microbiota were evaluated. We showed that male rats fed high-fat-sucrose diet developed gut microbiota dysbiosis, increased in body fat, decreased antioxidant activity, decreased brain neuropeptide Y, increased the number of astrocytes and activated microglia, along with reduced spine density associated with deficits in working memory. Ingestion of a combination of nopal, soy protein, curcumin, and chia seed oil (bioactive foods) for three months was associated with an increase in a cluster of bacteria with anti-inflammatory capacity, a decrease in serum LPS levels and an increase in serum eicosapentaenoic acid (EPA) with neuroprotective properties. In the liver, ingestion of bioactive food significantly increased antioxidant enzymes, decreased lipogenesis, reduced inflammation mediated by the TLR4-TNFα pathway along with a decrease in body fat, glucose intolerance, and metabolic inflexibility. Finally, neuroinflammation in the brain was reduced and working memory improved. Our study demonstrates that consumption of bioactive foods was associated with reduced liver, brain, and gut microbiota alterations in obese rats.
Source: Bioactive Foods Decrease Liver and Brain Alterations Induced by a High-Fat-Sucrose Diet through Restoration of Gut Microbiota and Antioxidant Enzymes. Nutrients. 2022 Jan; 14(1): 22. Published online 2021 Dec 22. doi: 10.3390/nu14010022
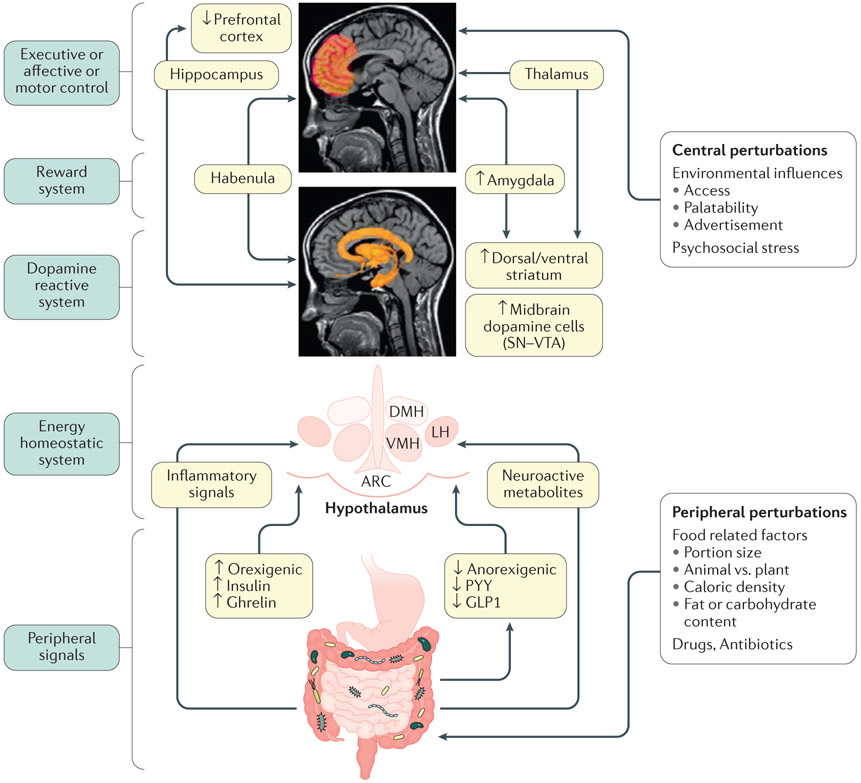
IMAGE: Model of brain–gut–microbiome interactions in ingestive behavior.
In the periphery, gut-generated and vagally transmitted orexogenic and anorexogenic signals interact with specific nuclei in the hypothalamus in the homeostatic regulation of food intake. Food-related factors interact with gut microorganisms and gut microbial metabolites modulate the release of orexogenic and anorexogenic peptides from enteroendocrine cells in the distal small intestine, shifting the balance between anorexogenic and orexogenic signaling in the hypothalamus. In addition, gut microorganisms can signal to the brain via inflammatory mediators (such as lipopolysaccharides) and neuroactive metabolites (such as tryptophan metabolites). Centrally, interactions between several brain networks, including the prefrontal cortex, the dopaminergic reward system and the sensorimotor system underlie the hedonic regulation of food intake. Several environmental influences such as food advertisements, food cues engage the extended reward system which can override the homeostatic control mechanisms. Exposure to visual and sensory cues, as well as psychosocial stress play important role in this process. Blue boxes on the left represent different parts of the BGM axis. Light green boxes in the center show mechanisms involved in altered BGM interactions in food addiction. Upward arrows show upregulation, downward arrows show downregulation. Modified with permission from Volkow et al. Biological Psychiatry 2013 EM: DONE
Source: Brain–gut–microbiome interactions in obesity and food addiction, Nature Reviews Gastroenterology & Hepatology
Normal eating behavior is coordinated by the tightly regulated balance between intestinal and extra-intestinal homeostatic and hedonic mechanisms. By contrast, food addiction represents a complex, maladaptive eating behavior that reflects alterations in brain–gut–microbiome (BGM) interactions and a shift of this balance towards hedonic mechanisms. Each component of the BGM axis has been implicated in the development of food addiction, with both brain to gut and gut to brain signaling playing a role. Early life influences can prime the infant gut microbiome and brain for food addiction, which might be further reinforced by increased antibiotic usage and dietary patterns throughout adulthood. The ubiquitous availability and marketing of inexpensive, highly palatable and calorie dense food can further shift this balance towards hedonic eating through both central (disruptions in dopaminergic signaling) and intestinal (vagal afferent function, metabolic toxaemia, systemic immune activation, changes to gut microbiome and metabolome) mechanisms. In this Review, we propose a systems biological model of BGM interactions, which incorporates published reports on food addiction, and provides novel insights into treatment targets aimed at each level of the BGM axis.
Source: Brain–gut–microbiome interactions in obesity and food addiction, Nature Reviews Gastroenterology & Hepatology
